We understand the stringent performance and quality requirements of the medical device industry
Our expertise includes highly regulated instruments such as anaesthesia and ventilation equipment, dialysis, blood pressure monitoring devices, and surgery.



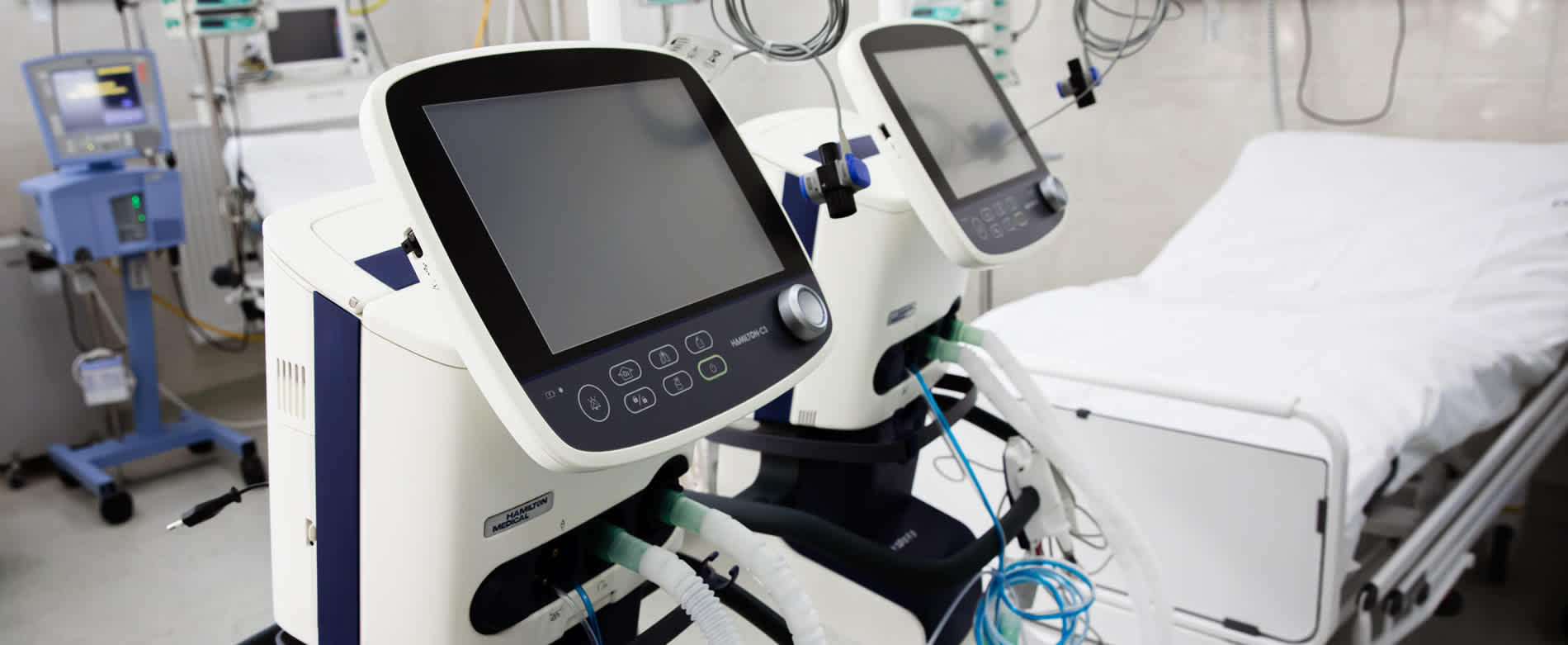
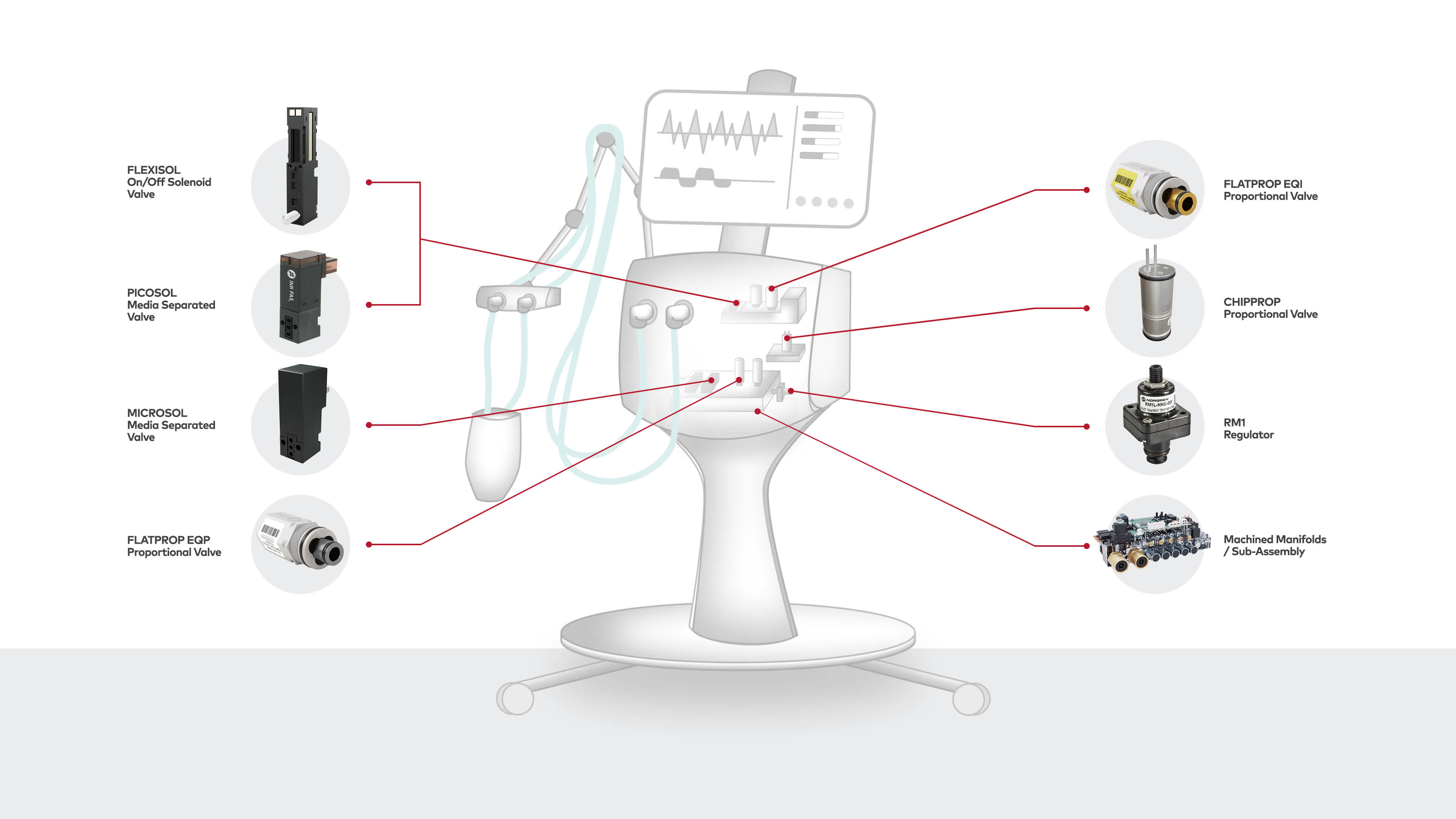
Ventilation machines
Artificial ventilation helps patients suffering from respiratory distress by assisting or stimulating their breathing, something they are not able to do themselves, and putting their life at risk.
There are various types of ventilators, adapted to all hospital requirements, as well as variants suited to home-care solutions. While the Intensive Care Units (ICU)/Critical Care instruments are stand-alone pieces of equipment, emergency/transport ventilators are small, portable, battery-powered units capable of covering the primary requirements.
Norgren's fluidic components play a crucial role in ICU ventilators. Proportional valves enable dosing of air and oxygen into patient lungs as well as PEEP control. Miniature on/off valves are ideal for auxiliary functions such as calibration of sensors.
All our valves for medical devices and diagnostic instrumentation applications are produced under ISO 13485. The 2020 Coronavirus pandemic infected millions of patients. For many, their natural lung function was severely affected, and the use of an ICU ventilator was required. Norgren is proud to supply all major ICU ventilator OEMs with solenoid proportional valves, on/off valves, pressure regulators and sub-assemblies, helping to save lives globally.
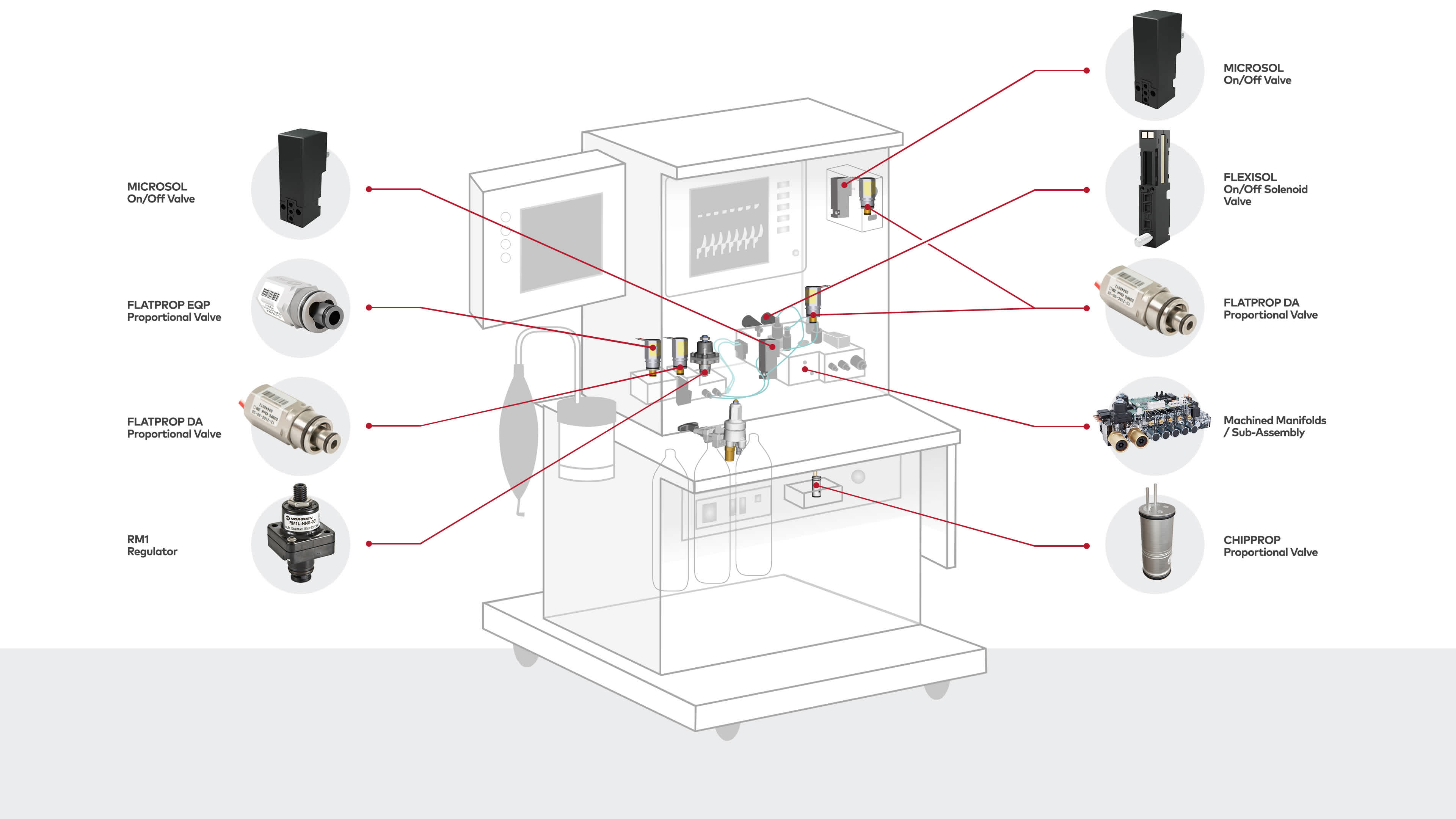
Anaesthesia machines
Anaesthesia machines provide a mixture of air, oxygen, and anaesthetic gases to the patient to allow medical procedures to be done without pain, and in some cases, without the patient being aware during the procedure.
In terms of fluidics, anaesthesia machines usually include all the main functions of a ventilator; with the addition of several functions such as anaesthesia gas dosing.
Our highly experienced engineering and production teams design and manufacture custom-made sub-assemblies including miniature on/off valves, miniature proportional valves, high flow proportional valves, pressure regulators, sensors and electronics on machined metal or plastic manifolds.
All our valves for medical devices and diagnostic instrumentation applications are produced under ISO 13485.
Dialysis
Dialysis is a medical treatment that is used to remove waste products and excess fluid from the blood of individuals with kidney failure.
There are two main types of dialysis: haemodialysis and peritoneal dialysis. Haemodialysis uses a machine to filter the blood, while peritoneal dialysis uses the lining of the abdominal cavity as a natural filter.
One of the main challenges that Original Equipment Manufacturers (OEMs) face in the dialysis industry is ensuring the reliability and safety of their equipment. Dialysis machines are used daily and must be able to function properly to keep patients safe.
Our engineers conduct extensive testing and quality control to ensure our equipment meets all relevant standards and regulations for quality and reliability. Examples of solutions include fully integrated valve manifolds for PD cyclers that offer compact and reliable solutions to minimise internal volumes and air consumption.
Surgery - Laparoscopy
Laparoscopy is a minimally invasive surgical procedure that allows a surgeon to access the inside of the abdomen and pelvis through small incisions in the skin.
OEMs working in the laparoscopy segment face several challenges. Laparoscopic equipment is subject to strict regulatory requirements, such as FDA clearance, and it is also critical that products are compatible with the latest surgical techniques.
To ensure that OEMs comply with all relevant standards and regulations, our products are designed and manufactured to the highest quality requirements and ISO 13485. As your trusted partner, Norgren can co-develop complete gas control subassemblies that enable the accurate and reliable control of gas used in surgical procedures.
Our manufacturing facilities offer protocols and processes designed to assist with the rigorous design, cleanliness, and packaging standards set forth by global governing agencies such as the FDA, EMA, and WHO. Partnering with us to design and/or manufacture a subassembly or source components for your product, means we collaborate with you on your requirements and specifications.
We have an extensive fluidic product portfolio and are solution providers for individual components and assemblies. From our rapid 3D printed prototyping, to repeat validation runs, our world class manufacturing facilities focus on products with the highest level of repeatability and accuracy.